Continuous monitoring of iron burden is essential in patients with TDT2,3
In patients with TDT, lifelong regular red blood cell (RBC) transfusions enable survival but lead to iron overload and treatment-related complications.1 The introduction of effective iron chelation therapy has significantly improved survival and quality of life for people living with TDT. However, even present-day chelators cannot completely prevent iron overload.1,3
In a 2018-2019 real-world, observational study* of 165 patients with TDT conducted in the UK, a subset of patients exhibited significant iron overload while being managed and monitored in a specialist center. Despite this level of care and utilizing iron chelation therapy, these patients experienced iron overload.4
*This study was funded by bluebird bio, who were involved in study design, interpretation of the data, and the submission of the paper for publication.
The 2021 Guidelines for the Management of TDT, published by the Thalassaemia International Federation (TIF), recommend starting iron chelation therapy when patients2:
OR
10 TO 20 TRANSFUSIONS
OR
LEVEL > 1000 ng/mL
Iron tissue uptake can lead to multi-organ damage2
Liver
Hepatic disease is becoming a leading cause of mortality as cardiac-related mortality declines due to advances in monitoring and chelation treatment.
- Excess iron is primarily stored in the liver and can reach clinically critical levels
Endocrine glands
Endocrine glands can be affected, resulting in conditions such as hypogonadotropic hypogonadism. Growth hormone deficiency can occur despite effective chelation therapy, due to iron deposition in the pituitary gland.
Heart
Symptomatic cardiac arrhythmias associated with myocardial iron overload pose significant clinical risk in older patients.
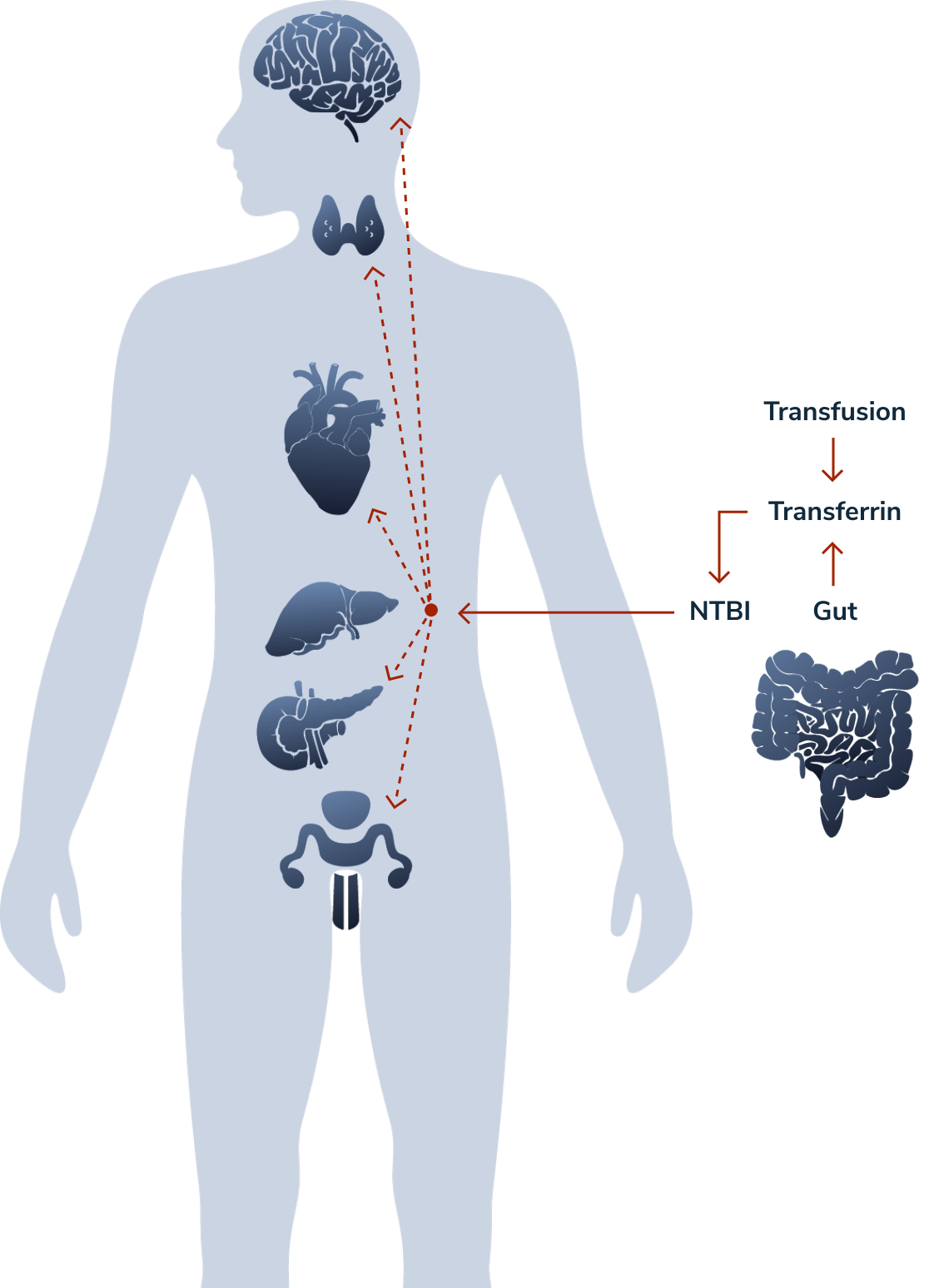
ALT = alanine transaminase
AST = aspartate transaminase
NTBI = non-transferrin-bound iron
Adapted from Guidelines for the Management of
Transfusion Dependent Thalassaemia (TDT). 4th ed.
Thalassaemia International Federation. 2021.
ALT = alanine transaminase
AST = aspartate transaminase
NTBI = non-transferrin-bound iron
Adapted from Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT). 4th ed. Thalassaemia International Federation. 2021.
ALT = alanine transaminase
AST = aspartate transaminase
NTBI = non-transferrin-bound iron
Adapted from Guidelines for the Management of
Transfusion Dependent Thalassaemia (TDT). 4th ed.
Thalassaemia International Federation. 2021.
ALT = alanine transaminase
AST = aspartate transaminase
NTBI = non-transferrin-bound iron
Adapted from Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT). 4th ed. Thalassaemia International Federation. 2021.
Transfusions temporarily relieve symptoms of anemia, but are associated with additional risks beyond iron overload
Additional risks include3,10:
- Infection
- Alloimmunity
- Transfusion-related acute lung injury
- Allergic, febrile, and delayed hemolytic reactions
Because of several technological advances including nucleic acid testing and improved donor selection program, blood safety has improved dramatically in recent years: the risk of contracting a viral infection is now reported to be less than 1 in a million blood transfusions.11